Low-density lipoprotein (LDL) cholesterol, long dubbed the “bad cholesterol,” has been at the center of cardiovascular medicine for decades. Its role in atherosclerosis and heart disease is well-documented, yet the molecular intricacies of its structure and function have remained somewhat elusive—until now. Thanks to revolutionary advances in cryo-electron microscopy (cryo-EM), artificial intelligence (AI)-driven modeling, and lipidomics, scientists have finally deciphered key aspects of LDL’s architecture and its complex interaction with cellular receptors. These discoveries are not merely academic—they offer transformative potential for how we diagnose, treat, and even prevent cardiovascular disease.
This article delves into the latest structural insights surrounding LDL cholesterol and the LDL receptor (LDLR), exploring what these findings mean for patients, particularly those with familial hypercholesterolemia (FH) and those at high risk for atherosclerosis. From enhancing genetic diagnostics to paving the way for next-generation therapies, this emerging frontier in lipid biology is poised to reshape cardiovascular care as we know it.
Table of Contents
The Role of LDL in Cholesterol Transport and Disease
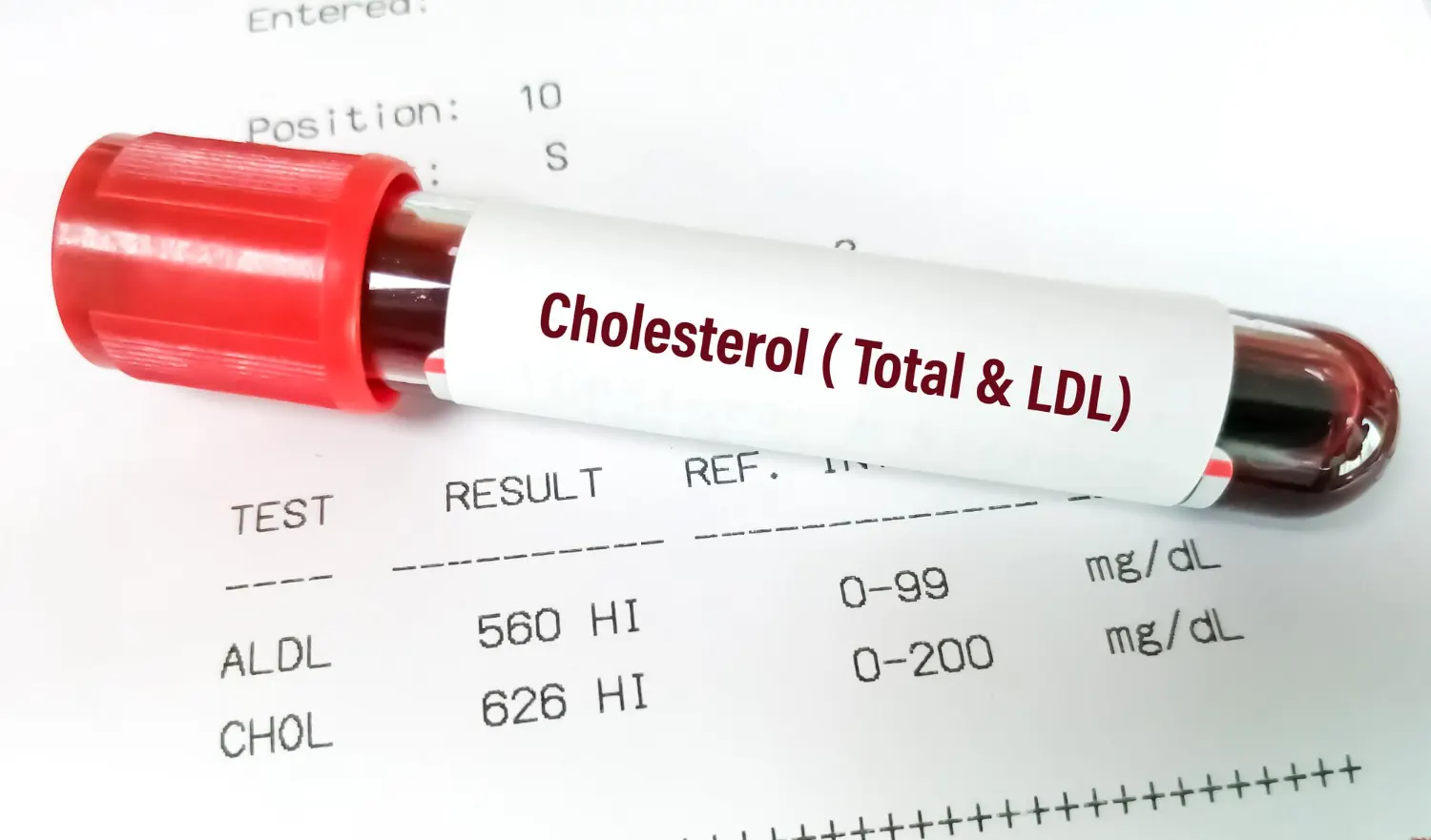
LDL particles are essential couriers of cholesterol in the human body. They transport cholesterol from the liver to peripheral tissues, where it is used for critical cellular functions such as membrane synthesis and steroid hormone production. Each LDL particle is primarily composed of a core of cholesterol esters and triglycerides, surrounded by phospholipids and a single copy of apolipoprotein B100 (apoB100), the protein that governs LDL’s structural integrity and receptor binding.
However, an excess of LDL in the bloodstream can lead to cholesterol deposition in arterial walls—a process known as atherogenesis. Over time, this buildup forms plaques that narrow arteries, restrict blood flow, and elevate the risk of heart attacks and strokes. LDL cholesterol is thus a key modifiable risk factor in the global battle against cardiovascular disease, the leading cause of mortality worldwide.
The clearance of LDL from circulation relies heavily on its interaction with LDLR on the surface of liver and peripheral cells. Through receptor-mediated endocytosis, LDL is internalized and broken down, allowing cells to absorb its cholesterol payload. Despite decades of understanding this general mechanism, the precise molecular details of how LDL binds to its receptor remained obscure due to technical challenges associated with imaging such a large and dynamic complex—until recent innovations changed the landscape.
A Molecular Breakthrough: High-Resolution Imaging of LDL and LDLR
The structural elucidation of the LDL particle, and in particular its interaction with LDLR, represents a landmark achievement in biomedical science. Using cryo-EM in conjunction with AlphaFold, a protein structure prediction tool developed by DeepMind, researchers have now visualized the LDL particle at subnanometer resolution. These studies, published in Nature and supported by the National Institutes of Health (NIH), have provided a complete 3D view of apoB100 in its native environment—wrapped around the lipid core of LDL like a protective belt.
Importantly, the research also revealed that LDL receptors do not act alone. Rather than binding one LDL particle at a time as previously assumed, LDLRs function as dimers, with the capacity to engage two LDL particles simultaneously. This dimerization greatly enhances the efficiency of LDL uptake and has significant implications for understanding how mutations in either apoB100 or LDLR can disrupt this process.
ApoB100 and LDLR: An Intricate Binding Mechanism
ApoB100 is one of the largest and most structurally complex proteins encoded by the human genome. Its amphipathic (both water- and lipid-loving) regions enable it to stabilize LDL particles and interface precisely with LDLR. Structural analysis has identified two major binding sites on apoB100 for LDLR:
- Ligand-binding domain interaction: The primary contact occurs through LDLR’s complement-type repeats, which latch onto electrostatic patches along the β-sheet belt of apoB100.
- β-propeller domain interaction: A secondary, stabilizing connection occurs between the N-terminal region of apoB100 and the β-propeller domain of LDLR.
This dual binding mechanism ensures high-affinity and high-specificity recognition. Mutations that alter either of these interfaces can severely impair LDL clearance, contributing to pathologies such as familial hypercholesterolemia.
Familial Hypercholesterolemia: Structural Clarity Meets Clinical Urgency
Familial hypercholesterolemia (FH) is a genetic disorder affecting approximately 1 in 250 individuals globally. It is characterized by persistently elevated LDL cholesterol levels from an early age, often resulting in premature atherosclerotic cardiovascular disease (ASCVD). Traditionally, FH has been linked to mutations in the LDLR, APOB, and PCSK9 genes. However, not all mutations were well understood—until now.
The mapping of pathogenic variants onto the newly resolved LDL-LDLR interaction domains offers unprecedented clarity. For example, mutations in APOB that disrupt the ligand-binding domain interface can now be directly linked to impaired receptor binding, providing a structural rationale for clinical phenotypes observed in FH patients. Similarly, variants in LDLR that impair dimerization or receptor folding have also been identified, giving geneticists and clinicians more precise tools for diagnosis.
This has immediate ramifications for clinical practice. Genetic screening can now target structurally critical regions with greater confidence, reducing false negatives and enabling earlier intervention for at-risk individuals.
Table: Impact of Structural Mutations on LDL-LDLR Function
| Mutation Location | Affected Gene | Structural Impact | Functional Consequence | Clinical Relevance |
|---|---|---|---|---|
| ApoB100 β-belt | APOB | Disrupted binding surface | Impaired LDLR recognition | Familial Hypercholesterolemia |
| LDLR β-propeller | LDLR | Loss of stabilizing interface | Ineffective endocytosis | Severe FH phenotype |
| LDLR Dimer Interface | LDLR | Prevents dimerization | Reduced LDL uptake efficiency | Intermediate FH risk |
| ApoB100 N-terminal | APOB | Weakens secondary binding | Decreased receptor affinity | Mild LDL elevation |
Therapeutic Implications: Targeting LDL-LDLR Interactions
The elucidation of LDL’s molecular architecture is not merely a scientific victory—it’s a clinical game changer. By revealing the specific domains responsible for receptor binding, researchers can now explore targeted interventions that mimic, enhance, or stabilize these interactions.
Emerging strategies include:
- Monoclonal antibodies or peptides that stabilize apoB100-LDLR binding.
- Small-molecule modulators designed to enhance LDLR dimerization and recycling.
- Gene therapy approaches using CRISPR/Cas9 to correct single-point mutations in APOB or LDLR.
These strategies complement existing pharmacological treatments and may provide alternatives for patients who are statin-intolerant or who fail to achieve target LDL levels despite maximal therapy.
Current Treatment Landscape: A Comparative Overview
The current arsenal for LDL-lowering therapy includes statins, ezetimibe, PCSK9 inhibitors, and newer agents like bempedoic acid. The choice of therapy depends on a patient’s risk profile, tolerance, and genetic background. Structural biology is enhancing this selection process by enabling personalized treatment plans rooted in molecular data.
Table: LDL-Lowering Therapies and Their Mechanisms
| Therapy Type | Mechanism of Action | LDL Reduction (%) | Ideal Use Case |
|---|---|---|---|
| Statins | Inhibit HMG-CoA reductase, upregulate LDLR | 30–60% | First-line for most patients |
| Ezetimibe | Blocks intestinal cholesterol absorption | 15–20% | Add-on for statin-intolerant patients |
| PCSK9 Inhibitors | Prevent LDLR degradation, boost recycling | 50–70% | High-risk or FH patients |
| Bempedoic Acid | Inhibits ATP-citrate lyase upstream of statins | 15–25% | Statin-intolerant or moderate-risk users |
| Gene Therapies (emerging) | Target APOB, LDLR, PCSK9 genes | Up to 80% (in trials) | FH patients with known pathogenic variants |
Beyond LDL: Lipoprotein(a) and Other Risk Factors
Lipoprotein(a), or Lp(a), is another lipoprotein particle gaining attention in cardiology circles. Similar to LDL in structure but containing an additional protein—apolipoprotein(a)—Lp(a) contributes independently to ASCVD risk. Importantly, its levels are largely genetically determined and unaffected by lifestyle.
High-resolution structural studies of LDL are now being adapted to explore Lp(a), which could accelerate the development of targeted therapies. This is crucial given that elevated Lp(a) often coexists with high LDL and may explain residual cardiovascular risk in otherwise well-managed patients.
Looking Ahead: The Era of Precision Lipidology
As the tools of structural biology become more refined, the field of lipidology is entering a precision era. Cryo-EM, AI modeling, and integrative genomics are converging to provide a unified framework for understanding lipoprotein function and dysfunction. This holds promise not just for cardiovascular medicine but for broader metabolic and inflammatory diseases linked to lipoproteins.
Researchers are now applying these technologies to VLDL, HDL, and chylomicrons—other lipoprotein particles involved in lipid transport and disease pathogenesis. The hope is that similar breakthroughs will unlock novel therapeutic targets across a spectrum of lipid disorders.
Conclusion: A New Frontier for Cardiovascular Care
The high-resolution structural mapping of LDL cholesterol and its receptor represents a transformative advance in our understanding of lipid biology. What was once a black box is now a blueprint—one that clinicians, geneticists, and pharmaceutical developers can use to craft more effective, personalized, and preventative cardiovascular care strategies.
For patients, particularly those with familial hypercholesterolemia or treatment-resistant dyslipidemia, this knowledge translates into earlier diagnoses, more precise treatments, and greater hope for a future free from premature cardiovascular events. As research continues to unfold, the insights gleaned from LDL’s structure may well serve as the foundation for a new chapter in precision medicine.
References
- NIH Research Matters. (2025). Lipoprotein structure gives insights into “bad” cholesterol. https://www.nih.gov/news-events/nih-research-matters/lipoprotein-structure-gives-insights-into-bad-cholesterol
- Tashko, G. (2024). Cholesterol Mysteries: How ApoB100 and LDL Receptors Bind. https://www.linkedin.com/pulse/cholesterol-mysteries-how-apob100-ldl-receptors-bind-gerti-tashko-md-f3khe
- Virani, S. S., et al. (2025). Independence of Lipoprotein(a) and LDL for ASCVD Risk. Circulation. https://www.ahajournals.org/doi/10.1161/CIRCULATIONAHA.124.069556
- Emerging Therapies for ASCVD. (2023). PMC. https://pmc.ncbi.nlm.nih.gov/articles/PMC10178593/
- NIH News Releases. (2024). NIH research reveals new insights about ‘bad’ cholesterol. https://www.nih.gov/news-events/news-releases/nih-research-reveals-new-insights-about-how-bad-cholesterol-works-body
- News-Medical. (2024). Key to LDL clearance uncovered. https://www.news-medical.net/news/20241212/Key-to-LDL-clearance-uncovered-Structural-details-of-apoB100-LDLR-binding-emerge.aspx
- Nature. (2024). Structure of apolipoprotein B100. https://www.nature.com/articles/s41586-024-08467-w


0 Comments